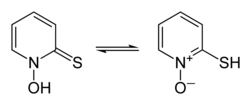
 Interconversion of pyrithione tautomers
thione form on the left, thiol form on the right | |
| Names | |
|---|---|
| Preferred IUPAC name
1-Hydroxy-2(1H)-pyridinethione (thione) 2-Pyridinethiol 1-oxide (thiol) | |
| Other names
Omadine
thione: 1-Hydroxypyridine-2-thione N-Hydroxypyridine-2-thione thiol: 2-Mercaptopyridine monoxide 2-Mercaptopyridine N-oxide 2-Mercaptopyridine 1-oxide | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| 109936 | |
| ChEBI |
|
| ChEMBL |
|
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.013.027 |
| EC Number |
|
| 913415 | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H5NOS | |
| Molar mass | 127.16 g·mol−1 |
| Appearance | Beige crystalline powder |
| Melting point | 70 to 73 °C (158 to 163 °F; 343 to 346 K) |
| 2.5 g L−1 at 20 °C | |
| Solubility | Soluble: benzene, chloroform, dichloromethane, dimethylformamide, dimethylsulfoxide, ethyl acetate[1] Slightly soluble: diethyl ether, ethanol, methyl tert-butyl ether, tetrahydrofuran[1] |
| Acidity (pKa) | −1.95 (proton addition), 4.6[2][3] |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H301, H315, H319, H335 | |
| P261, P264, P270, P271, P280, P301+P310, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pyrithione is the common name of an organosulfur compound with molecular formula C
5H
5NOS, chosen as an abbreviation of pyridinethione, and found in the Persian shallot.[4] It exists as a pair of tautomers, the major form being the thione 1-hydroxy-2(1H)-pyridinethione and the minor form being the thiol 2-mercaptopyridine N-oxide; it crystallises in the thione form.[5] It is usually prepared from either 2-bromopyridine,[1] 2-chloropyridine,[6][7] or 2-chloropyridine N-oxide,[8] and is commercially available as both the neutral compound and its sodium salt.[1] It is used to prepare zinc pyrithione,[9][10] which is used primarily to treat dandruff and seborrhoeic dermatitis in medicated shampoos,[11][12] though is also an anti-fouling agent in paints.[13]
- ^ a b c d Cite error: The named reference
eEROS-Pyrithionewas invoked but never defined (see the help page). - ^ Cite error: The named reference
pKawas invoked but never defined (see the help page). - ^ Cite error: The named reference
pKa2was invoked but never defined (see the help page). - ^ Cite error: The named reference
PersianShallotwas invoked but never defined (see the help page). - ^ Cite error: The named reference
SolidThionewas invoked but never defined (see the help page). - ^ Cite error: The named reference
2-mercaptopyridinewas invoked but never defined (see the help page). - ^ Cite error: The named reference
UroniumChloropyridinewas invoked but never defined (see the help page). - ^ Cite error: The named reference
Romppwas invoked but never defined (see the help page). - ^ Cite error: The named reference
ZnP2patent1was invoked but never defined (see the help page). - ^ Cite error: The named reference
ZnP2patent2was invoked but never defined (see the help page). - ^ Cite error: The named reference
ZnDermatologywas invoked but never defined (see the help page). - ^ Cite error: The named reference
SebDermwas invoked but never defined (see the help page). - ^ Cite error: The named reference
ACSwas invoked but never defined (see the help page).
