
Back حمض البيروفسفوريك Arabic پیروفوسفوریک اسید AZB Pirofosforna kiselina BS Àcid difosfòric Catalan Diphosphorsäure German Pirofosfata acido EO Ácido difosfórico Spanish پیروفسفریک اسید FA Pyrofosforihappo Finnish Acide pyrophosphorique French
| |

| |
| Names | |
|---|---|
| IUPAC names
Diphosphoric acid
μ-oxido-bis(dihydroxidooxidophosphorus) | |
| Other names
Pyrophosphoric acid
Phosphonophosphoric acid Phosphono dihydrogenphosphate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.017.795 |
| EC Number |
|
| 82619 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
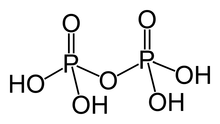
| H4P2O7 | |
| Molar mass | 177.97 g/mol |
| Melting point | 71.5 °C (160.7 °F; 344.6 K) |
| Extremely soluble | |
| Solubility | Very soluble in alcohol, ether |
| Conjugate base | Pyrophosphate |
| Hazards | |
| GHS labelling:[1] | |
 
| |
| Danger | |
| H302, H314 | |
| P260, P264, P264+P265, P270, P280, P301+P317, P301+P330+P331, P302+P361+P354, P304+P340, P305+P354+P338, P316, P317, P321, P330, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pyrophosphoric acid, also known as diphosphoric acid, is the inorganic compound with the formula H4P2O7 or, more descriptively, [(HO)2P(O)]2O. Colorless and odorless, it is soluble in water, diethyl ether, and ethyl alcohol. The anhydrous acid crystallizes in two polymorphs, which melt at 54.3 and 71.5 °C. The compound is a component of polyphosphoric acid, an important source of phosphoric acid.[1] Anions, salts, and esters of pyrophosphoric acid are called pyrophosphates.