
Back ريبافيرين Arabic ریباویرین AZB Ribavirina Catalan Ribafirin CY Ribavirin German Ribavirina Spanish ریباویرین FA Ribaviriini Finnish Ribavirine French Ռիբավիրին HY
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌraɪbəˈvaɪrɪn/ RY-bə-VY-rin |
| Trade names | Copegus, Rebetol, Virazole, other[1] |
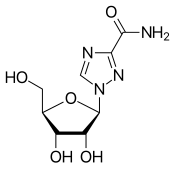
| Other names | 1-(β-D-Ribofuranosyl)-1"H"-1,2,4-triazole-3-carboxamide, tribavirin (BAN UK) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605018 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral, Inhalation |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 64%[5] |
| Protein binding | 0%[5] |
| Metabolism | liver and intracellularly[5] |
| Elimination half-life | 298 hours (multiple dose); 43.6 hours (single dose)[5] |
| Excretion | Urine (61%), faeces (12%)[5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.164.587 |
| Chemical and physical data | |
| Formula | C8H12N4O5 |
| Molar mass | 244.207 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 166 to 168 °C (331 to 334 °F) |
| |
| |
| (verify) | |
Ribavirin, also known as tribavirin, is an antiviral medication used to treat illness caused by respiratory syncytial virus (RSV) and hepatitis C virus (HCV) infections, as well as some viral hemorrhagic fevers.[1] For HCV, it is used in combination with other medications, such as simeprevir, sofosbuvir, peginterferon alfa-2b or peginterferon alfa-2a.[1] It can also be used for viral hemorrhagic fevers—specifically, for Lassa fever, Crimean–Congo hemorrhagic fever, and Hantavirus infections (with exceptions for Ebola or Marburg virus diseases).[1] Ribavirin is usually taken orally (by mouth) or inhaled.[1] Despite widespread usage, it has faced scrutiny in the 21st century because of lack of proven efficacy in treating viral infections for which it has been prescribed in the past.[6][7]
Its common side effects include fatigue, headache, nausea, fever, muscle pains, and an irritable mood.[1] Serious side effects include red blood cell breakdown, liver problems, and allergic reactions.[1] Its use during pregnancy can bring harm to the developing fetus.[1] Effective birth control is recommended for both males and females for at least seven months during and after use.[8] The mechanism of action of ribavirin is not entirely clear.[1]
Ribavirin was patented in 1971 and approved for medical use in 1986.[9] It is on the World Health Organization's List of Essential Medicines.[10] It is available as a generic medication.[1]
- ^ a b c d e f g h i j k "Ribavirin". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 8 December 2016.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ "Ribavirin (Ibavyr)". Catie. 2022. Retrieved 22 August 2022.
- ^ a b c d e "PRODUCT INFORMATION REBETOL (RIBAVIRIN) CAPSULES" (PDF). TGA eBusiness Services. Merck Sharp & Dohme (Australia) Pty Limited. 29 April 2013. Archived from the original on 16 August 2017. Retrieved 23 February 2014.
- ^ Cite error: The named reference
Safrin_2024was invoked but never defined (see the help page). - ^ Cite error: The named reference
Drugs.com_2023was invoked but never defined (see the help page). - ^ World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 177. hdl:10665/44053. ISBN 9789241547659.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 504. ISBN 9783527607495. Archived from the original on 2016-12-20.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.