
Back تنوآزونیک اسید AZB Tenuazonsäure German تنوآزونیک اسید FA Tenuazonsav Hungarian テヌアゾン酸 Japanese Tenuazonska kiselina SH Tenuazonska kiselina Serbian 细交链孢菌酮酸 Chinese
 | |
| Names | |
|---|---|
| Preferred IUPAC name
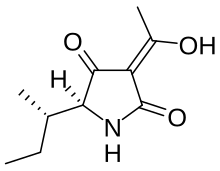
(5S)-3-Acetyl-5-[(2S)-butan-2-yl]-4-hydroxy-1,5-dihydro-2H-pyrrol-2-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.164.201 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H15NO3 | |
| Molar mass | 197.234 g·mol−1 |
| Appearance | White crystalline powder |
| Acidity (pKa) | 3.5 |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
* 182 mg kg−1 (Mice, ♂, oral)[1] |
| Pharmacology | |
| Ingested or Inhaled | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tenuazonic acid is a mycotoxin produced by Alternaria species.[3] It is a powerful eukaryotic protein synthesis inhibitor.[4] It is a tetrameric acid that is ubiquitous in biological environments and prevents the release of newly synthesized protein from the ribosome. Its toxicity is the highest among all Alternaria mycotoxins and has both phytotoxic and cytotoxic properties.[5] In 1991 Tenuazonic acid was reported to inhibit skin tumor promotion in mice.[6]
- ^ a b Miller, F. A. et al.; Nature, 200 (1963), S. 1338–1339
- ^ Smith, E. R. et al.; Cancer Chemother. Rep. 52 (1968), S. 579–585.
- ^ Alisa D. Hocking (Editor), John I. Pitt (Editor) and Robert A. Samson (Editor): Advances in Food Mycology. Springer 2006; ISBN 978-0-387-28385-2; p. 23
- ^ Dilip K. Arora and Arora K. Arora: Fungal Biotechnology in Agricultural, Food, and Environmental Applications. Marcel Dekker Inc; illustrated edition 2003; ISBN 978-0-8247-4770-1; p. 336
- ^ Mikula, Hannes; Horkel, Ernst; Hans, Philipp; Hametner, Christian; Fröhlich, Johannes (2013-04-15). "Structure and tautomerism of tenuazonic acid--a synergetic computational and spectroscopic approach". Journal of Hazardous Materials. 250–251: 308–317. Bibcode:2013JHzM..250..308M. doi:10.1016/j.jhazmat.2013.02.006. ISSN 1873-3336. PMID 23474405.
- ^ Tenuazonic acid page from Fermentek