
Back هيدروكسيد رباعي ميثيل الأمونيوم Arabic تترامتیلآمونیوم هیدروکسید AZB Hidròxid de tetrametilamoni Catalan Tetramethylammoniumhydroxid German Tetrametilamonia hidroksido EO تترامتیلآمونیوم هیدروکسید FA Tetrametyyliammoniumhydroksidi Finnish Idrossido di tetrametilammonio Italian 水酸化テトラメチルアンモニウム Japanese Tetramethylammoniumhydroxide Dutch
| |

| |
| Names | |
|---|---|
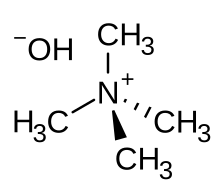
| IUPAC name
N,N,N-Trimethylmethanaminium hydroxide
| |
| Other names
tetramethylammonium hydroxide
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.000.803 |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H13NO | |
| Molar mass | 91.154 g·mol−1 |
| Density | ~ 1.015 g/cm3 (20-25% aqueous solution) |
| Melting point | 67 °C (153 °F; 340 K) (pentahydrate) |
| Boiling point | decomposes |
| high | |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger[1] | |
| H300, H311, H314[1] | |
| P260, P264, P270, P280, P301+P310, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P322, P361, P363, P405, P501[1] | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | Sigma-Aldrich MSDS for TMAH·5H2O |
| Related compounds | |
Other anions
|
tetramethylammonium chloride |
Other cations
|
tetraethylammonium hydroxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tetramethylammonium hydroxide (TMAH or TMAOH) is a quaternary ammonium salt with molecular formula N(CH3)4+ OH−. It is commonly encountered in form of concentrated solutions in water or methanol. TMAH in solid state and its aqueous solutions are all colorless, but may be yellowish if impure. Although TMAH has virtually no odor when pure, samples often have a strong fishy smell due to presence of trimethylamine which is a common impurity. TMAH has several diverse industrial and research applications.
- ^ a b c d Sigma-Aldrich Co., Tetramethylammonium hydroxide pentahydrate. Retrieved on 2015-04-06.
