
Back كالسيتريول Arabic کلسیتریول AZB Kalcitriol BS Calcitriol Catalan Kalcitriol Czech Calcitriol CY Calcitriol German ކެލްސިޓްރިއޯލް DV Calcitriol English Calcitriol Spanish
| |||
| (IUPAC) ime | |||
|---|---|---|---|
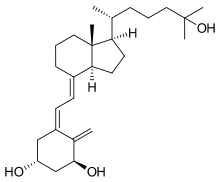
| (1R,3S)-5-[2-[(1R,3aR,7aS)-1-[(2R)-6-hidroksi-6-metil-heptan-2-il]-7a-metil-2,3,3a,5,6,7-heksahidro-1H- inden-4-iliden]etiliden]-4-metiliden-cikloheksan-1,3-diol | |||
| Klinički podaci | |||
| Robne marke | Rocaltrol, Calcijex, Decostriol | ||
| MedlinePlus | a682335 | ||
| Identifikatori | |||
| CAS broj | 32222-06-3 | ||
| ATC kod | A11CC04 D05AX03 | ||
| PubChem[1][2] | 134070 | ||
| DrugBank | DB00136 | ||
| ChemSpider[3] | 4941667 | ||
| UNII | FXC9231JVH | ||
| ChEBI | CHEBI:17823 | ||
| ChEMBL[4] | CHEMBL846 | ||
| Hemijski podaci | |||
| Formula | C27H44O3 | ||
| Mol. masa | 416,64 g/mol | ||
| SMILES | eMolekuli & PubHem | ||
| |||
| Farmakokinetički podaci | |||
| Metabolizam | Renalno | ||
| Poluvreme eliminacije | 5–8 sata | ||
| Izlučivanje | Renal | ||
| Farmakoinformacioni podaci | |||
| Trudnoća | ? | ||
| Pravni status | S4 (Au), POM (UK) | ||
| Način primene | Oralno, IV, topikalno | ||
Kalcitriol (1,25-dihidroksiholekalciferol, 1,25-dihidroksivitamin D3) je hormonski aktivna forma vitamina D sa tri hidroksilne grupe (1,25-(OH)2D3 ili 1,25(OH)2D).[5][6] On povišava nivo kalcijuma (Ca2+) u krvi putem (1) povećanja unosa kalcijuma iz creva u krv, i (2) mogućeg povišenja otpuštanja kalcijuma u krv iz kostiju.[7]
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ "Nomenclature of Vitamin D. Recommendations 1981. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN)" reproduced at the Queen Mary, University of London website., Pristupljeno 21. 3. 2010.
- ↑ Holick, MF; Schnoes, HK; Deluca, HF; Suda, T; Cousins, RJ (1971). „Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine”. Biochemistry 10 (14): 2799–804. DOI:10.1021/bi00790a023. PMID 4326883.
- ↑ Donald Voet, Judith G. Voet (2005). „Biomolecules, mechanisms of enzyme action, and metabolism”. Biochemistry (3 izd.). Wiley. str. 663-664. ISBN 978-0-471-19350-0.