
Back حمض الليبويك Arabic Lipoik turşu AZ لیپوئیک اسید AZB Àcid lipoic Catalan Kyselina lipoová Czech Liponsäure German Lipoic acid English Ácido lipoico Spanish Lipoehape ET لیپوئیک اسید FA
| Lipoinska kiselina | |||
|---|---|---|---|
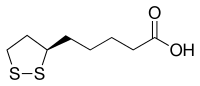
| |||

| |||
| IUPAC ime |
| ||
| Drugi nazivi | α-Lipoinska kiselina (alfa lipoinska kiselina); Tioktinska kiselina; 6,8-Ditiooktanoinska kiselina | ||
| Identifikacija | |||
| CAS registarski broj | 1200-22-2 | ||
| PubChem[1][2] | 6112 | ||
| ChemSpider[3] | 5886 | ||
| DrugBank | DB00166 | ||
| KEGG[4] | |||
| MeSH | |||
| ChEBI | 30314 | ||
| ChEMBL[5] | CHEMBL134342 | ||
| ATC code | A16 | ||
| Jmol-3D slike | Slika 1 | ||
| |||
| |||
| Svojstva | |||
| Molekulska formula | C8H14O2S2 | ||
| Molarna masa | 206.33 g mol−1 | ||
| Agregatno stanje | Žuti igličasti kristali | ||
| Rastvorljivost u vodi | Rastvorna kao natrijumova so | ||
| Rastvorljivost u etanol | Rastvorna | ||
| Farmakologija | |||
| Bioraspoloživost | 30% (oralno)[6] | ||
| Srodna jedinjenja | |||
| Srodna jedinjenja | Lipoamid Asparagusna kiselina | ||
|
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje (25 °C, 100 kPa) materijala | |||
| Infobox references | |||
Lipoinska kiselina (LA, α-lipoinska kiselina[7] i alfa lipoinska kiselina, ALA[8]) je organosumporno jedinjenje izvedeno iz oktanoinske kiseline. Lipoinska kiselina sadrži dva atoma sumpora (između C6 i C8) povezana disulfidnom vezom i stoga se smatra oksidovanim molekulom (mada atomi sumpora mogu da postoje u višim oksidacionim stanjima). Atom ugljenika u C6 poziciji je hiralan, te se molekul se javlja u obliku dva enantiomera (R)-(+)-lipoinska kiselina (RLA) i (S)-(-)-lipoinska kiselina (SLA), kao i u obliku racemske smeše (R/S)-lipoinske kiseline (R/S-LA). Jedino se (R)-(+)-enantiomer javlja u prirodi i on je esencijalni kofaktor za četiri mitohondrijska enzimska kompleksa.[9] Endogeno sintetisana RLA je esencijalna za život i aerobni metabolizam.
RLA i R/S-LA su dostupne na slobodno kao nutricioni suplementi. One su u prehrambenoj i kliničkoj upotrebi od 1950-tih za razne bolesti i poremećaje. Lipoinska kiselina je žuta čvrsta materija.
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ Teichert J, Hermann R, Ruus P, Preiss R (November 2003). „Plasma kinetics, metabolism, and urinary excretion of alpha-lipoic acid following oral administration in healthy volunteers”. J Clin Pharmacol 43 (11): 1257–67. DOI:10.1177/0091270003258654. PMID 14551180.
- ↑ Petersen Shay, K, Moreau, RF, Smith, EJ, Hagen, TM (June 2008). „Is alpha-lipoic acid a scavenger of reactive oxygen species in vivo? Evidence for its initiation of stress signaling pathways that promote endogenous antioxidant capacity”. IUBMB life 60 (6): 362–7. DOI:10.1002/iub.40. PMID 18409172.
- ↑ Reljanovic M, Reichel G, Rett K, et al. (September 1999). „Treatment of diabetic polyneuropathy with the antioxidant thioctic acid (alpha-lipoic acid): a two year multicenter randomized double-blind placebo-controlled trial (ALADIN II). Alpha Lipoic Acid in Diabetic Neuropathy”. Free Radic. Res. 31 (3): 171–9. PMID 10499773.
- ↑ Raddatz, G, Bisswanger, H (October 1997). „Receptor site and stereospecifity of dihydrolipoamide dehydrogenase for R- and S-lipoamide: a molecular modeling study”. Journal of Biotechnology 58 (2): 89–100. DOI:10.1016/S0168-1656(97)00135-1. PMID 9383983.