Back بورنيول Arabic Borneol AZ برنئول AZB Borneol Catalan Borneol Czech Borneol Danish Borneolo EO Borneol Spanish برنئول FA Borneoli Finnish
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
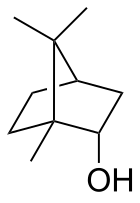
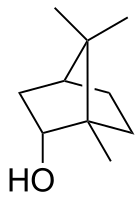
rel-(1R,2S,4R)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ol
| |||
| Other names
1,7,7-Trimethylbicyclo[2.2.1]heptan-2-endo-ol
endo-2-Bornanol, Borneo camphor | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.006.685 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| UN number | 1312 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H18O | |||
| Molar mass | 154.253 g·mol−1 | ||
| Appearance | colorless to white lumps | ||
| Odor | pungent, camphor-like | ||
| Density | 1.011 g/cm3 (20 °C)[1] | ||
| Melting point | 208 °C (406 °F; 481 K) | ||
| Boiling point | 213 °C (415 °F; 486 K) | ||
| slightly soluble (D-form) | |||
| Solubility | soluble in chloroform, ethanol, acetone, ether, benzene, toluene, decalin, tetralin | ||
| −1.26×10−4 cm3/mol | |||
| Hazards | |||
| GHS labelling: | |||

| |||
| Warning | |||
| H228 | |||
| P210, P240, P241, P280, P370+P378 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 65 °C (149 °F; 338 K) | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Related compounds
|
Bornane (hydrocarbon) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Borneol is a bicyclic organic compound and a terpene derivative. The hydroxyl group in this compound is placed in an endo position. The exo diastereomer is called isoborneol. Being chiral, borneol exists as enantiomers, both of which are found in nature: d-borneol (also written (+)-borneol) and l-borneol (or (−)-borneol).
- ^ Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton, Florida: CRC Press. p. 3.56. ISBN 0-8493-0486-5.