Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
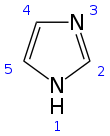
Imidazole
| |||
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1H-Imidazole[1] | |||
| Other names
1,3-Diazole
Glyoxaline (archaic) | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 103853 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.005.473 | ||
| EC Number |
| ||
| 1417 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 3263 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H4N2 | |||
| Molar mass | 68.077 g/mol | ||
| Appearance | White or pale yellow solid | ||
| Density | 1.23 g/cm3, solid | ||
| Melting point | 89 to 91 °C (192 to 196 °F; 362 to 364 K) | ||
| Boiling point | 256 °C (493 °F; 529 K) | ||
| 633 g/L | |||
| Acidity (pKa) | 6.95 (for the conjugate acid) [2] | ||
| UV-vis (λmax) | 206 nm | ||
| Structure | |||
| Monoclinic | |||
| Planar 5-membered ring | |||
| 3.61 D | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Corrosive | ||
| GHS labelling:[4] | |||
  
| |||
| Danger | |||
| H302, H314, H360D | |||
| P263, P270, P280, P301+P310, P305+P351+P338, P308+P313[3] | |||
| Flash point | 146 °C (295 °F; 419 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Imidazole (ImH) is an organic compound with the formula (CH)3(NH)N. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. It can be classified as a heterocycle, specifically as a diazole.
Many natural products, especially alkaloids, contain the imidazole ring. These imidazoles share the 1,3-C3N2 ring but feature varied substituents. This ring system is present in important biological building blocks, such as histidine and the related hormone histamine. Many drugs contain an imidazole ring, such as certain antifungal drugs, the nitroimidazole series of antibiotics, and the sedative midazolam.[5][6][7][8][9]
When fused to a pyrimidine ring, it forms a purine, which is the most widely occurring nitrogen-containing heterocycle in nature.[10]
The name "imidazole" was coined in 1887 by the German chemist Arthur Rudolf Hantzsch (1857–1935).[11]
- ^ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 140. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Walba, H.; Isensee, R. W. (1961). "Acidity constants of some arylimidazoles and their cations". J. Org. Chem. 26 (8): 2789–2791. doi:10.1021/jo01066a039.
- ^ "Imidazole". molekula.com. Molekula Group. Archived from the original on 2018-10-19. Retrieved 2018-10-19.
- ^ "Imidazole". pubchem.ncbi.nlm.nih.gov. Archived from the original on 10 May 2023. Retrieved 17 February 2024.
- ^ Karitzky, A. R.; Rees, C.W.R.; Scriven, E.F.V. (1984). Comprehensive Heterocyclic Chemistry. Vol. 5. pp. 469–498. ISBN 978-0-08-042072-1.
- ^ Grimmett, M. Ross (1997). Imidazole and Benzimidazole Synthesis. Academic Press. ISBN 978-0-08-053445-9.
- ^ Brown, E. G. (1998). Ring Nitrogen and Key Biomolecules. Kluwer Academic Press. ISBN 978-94-011-4906-8.
- ^ Pozharskii, A. F.; et al. (1997). Heterocycles in Life and Society. John Wiley & Sons. ISBN 978-0-471-96033-1.
- ^ Gilchrist, T. L. (1985). Heterocyclic Chemistry. Bath Press. ISBN 978-0-582-01421-3.
- ^ Rosemeyer, H. (2004). "The Chemodiversity of Purine as a Constituent of Natural Products". Chemistry & Biodiversity. 1 (3): 361–401. doi:10.1002/cbdv.200490033. PMID 17191854. S2CID 12416667.
- ^ Hantzsch, A. and Weber, J. H. (1887) "Ueber Verbindungen des Thiazols (Pyridins der Thiophenreihe)" Archived 2020-05-30 at the Wayback Machine (On compounds of thiazole (pyridines of the thiophene series), Berichte der deutschen chemischen Gesellschaft, 20 : 3118–3132, see p. 3119. See also: Hantzsch, A. (1888) "Allegemeine Bemerkungen über Azole" Archived 2020-05-30 at the Wayback Machine (General observations about azoles), Annalen der Chemie, 249 : 1–6. Hantzsch proposed a reform of the nomenclature of azole compounds, including a proposal to call the heterocyclic ring C3H3(NH)N "imidazole"; see pp. 2 and 4.
Previous Page Next Page