Our website is made possible by displaying online advertisements to our visitors.
Please consider supporting us by disabling your ad blocker.
Dimercaprol
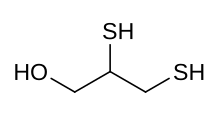 | |
 Skeletal formula and ball and stick model of dimercaprol | |
| Clinical data | |
|---|---|
| Trade names | BAL in Oil |
| Other names | 2,3-Dimercaptopropanol British Anti-Lewisite 2,3-Dithiopropanol 2,3-Dimercaptopropan-1-ol British antilewisite |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | intramuscular |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Excretion | Urine[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.394 |
| Chemical and physical data | |
| Formula | C3H8OS2 |
| Molar mass | 124.22 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.239 g/cm3 |
| Boiling point | 393 °C (739 °F) at 2.0 kPa |
| |
| |
Dimercaprol, also called British anti-Lewisite (BAL), is a medication used to treat acute poisoning by arsenic, mercury, gold, and lead.[3] It may also be used for antimony, thallium, or bismuth poisoning, although the evidence for those uses is not very strong.[3][4] It is given by injection into a muscle.[3]
Common side effects include high blood pressure, pain at the site of the injection, vomiting, and fever.[3] It is not recommended for people with peanut allergies as it is typically formulated as a suspension in peanut oil.[3] It is unclear if use in pregnancy is safe for the baby.[3] Dimercaprol is a chelator and works by binding with heavy metals.[3] It has a very pungent odor.
Dimercaprol was first made during World War II.[5] It is on the World Health Organization's List of Essential Medicines.[6]
- ^ Poisoning in Children. Jaypee Brothers Publishers. 2013. p. 70. ISBN 978-93-5025-773-9.
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 697. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
The prefixes 'mercapto' (–SH), and 'hydroseleno' or selenyl (–SeH), etc. are no longer recommended.
- ^ a b c d e f g "Dimercaprol". The American Society of Health-System Pharmacists. Archived from the original on 21 December 2016. Retrieved 8 December 2016.
- ^ World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 62. hdl:10665/44053. ISBN 978-92-4-154765-9.
- ^ Greenwood D (2008). "Antiprotozoal Agents". Antimicrobial Drugs: Chronicle of a Twentieth Century Medical Triumph. OUP Oxford. p. 281. ISBN 978-0-19-953484-5. Archived from the original on 2016-12-20.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
Previous Page Next Page


